Breaking the deadly cycle: Drug researchers tackle the malaria parasite at multiple life stages

Malaria, a mosquito-borne disease caused by the Plasmodium parasite, is a scourge in the developing world, impacting 200 million people and killing nearly 450,000 people each year, many of whom are children aged 5 and under. Although we already have preventative medicines in place, they simply aren't enough to combat the persistent parasite that is becoming increasingly drug-resistant.
Paul Carlier, a professor in the Department of Chemistry in the Virginia Tech College of Science and director of the Virginia Tech Center for Drug Discovery, and his colleagues received a $3.7 million grant from the National Institutes of Health to begin further testing of two inexpensive and effective antimalarial drug candidates that can kill Plasmodium parasites at three different stages of their development.
“The most effective drug for malaria at present is not safe for pregnant women, or children 5 years or less,” said Carlier, who is an affiliated faculty member of Virginia Tech’s Center for Emerging, Zoonotic, and Arthropod-borne Pathogens, an arm of the Fralin Life Sciences Institute. "Those are the populations that most need treatment, and yet, they are the most vulnerable to drug toxicity. Because of that limitation, and the parasite’s resistance to other existing drugs, we need to create better, safer drugs.”
Back in 2016, with the support of a two-year grant from the National Institutes of Health, the team had a scaffold, or a basic chemical structure, that they meticulously tweaked with various chemical modifications to see if it was more or less effective against malaria.
From the original compound MMV008138, they created two promising new antimalarial drug candidates, or compounds that have the potential to become a drug, called PRC1584 and PRC1590.
To understand malarial infection and how drugs can work against it, it is important to learn about the impressively complex life cycle of the Plasmodium parasite. The parasite’s war against the body takes place in three stages: the liver stage, the asexual blood stage, and the gametocyte, or sexual blood stage.
But before the parasite can even attack the body, it first infects a female Anopheles mosquito, the only mosquito species that can transmit malaria. When an unsuspecting mosquito feeds on a person infected with malaria, it sucks up a small amount of blood containing gametocytes that immediately transform into gametes, or parasitic eggs and sperm. After completing their whole life cycle within the insect, the fully grown parasites enter the mosquito's saliva and are ready for deployment.
After a human host is bitten by an infected mosquito, the parasites, which initially number less than one hundred, travel to the liver to multiply to build their infantry.
Then, like a hostile army overwhelming the field of battle, thousands of Plasmodium parasites invade the bloodstream and infect red blood cells (RBCs). Parasites within the blood stage are the target of most malaria medications since they induce the disease's clinical signs, such as fever, vomiting, and anemia. It is when the quantity of parasites in red blood cells reaches 100 million that symptoms occur. The parasites' rapid development is linked to their asexual reproduction, which does not require eggs or sperm to reproduce.
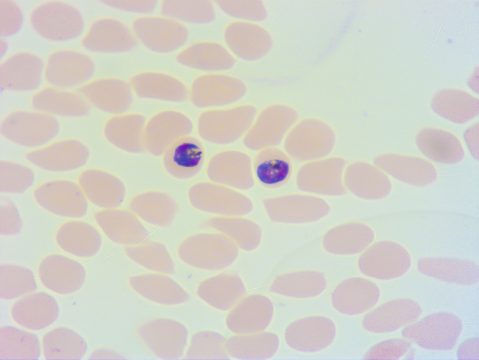
In the bloodstream, the parasites can also develop into a sexual form called the gametocyte stage. In this form, the parasites produce parasitic eggs and sperm so that they can be transmitted to the next Anopheles mosquito vector.
“If you have this sexual form circulating in your blood, and you get bitten by the mosquito, that’s the only life stage that can continue the cycle and infect the mosquitoes,” said Carlier. “The ideal drug would break the cycle by targeting all three life stages: liver stage for prevention, asexual blood stage for therapy, and gametocytes to block disease transmission.”
The ultimate goal is to create an antimalarial that would have these characteristics, and also cure severe malaria with a single oral dose.
To achieve these goals, Carlier will continue his collaboration with Maria Belen Cassera, an associate professor of biochemistry and molecular biology and faculty member of the Center for Tropical & Emerging Global Diseases at the University of Georgia, and Max Totrov, a computational chemist at Molsoft, a leading provider of tools, databases and consulting services in the area of structural proteomics, bioinformatics, and rational drug design.
After Carlier, a medicinal chemist, prepares new variations of the lead antimalarial compounds, they will be sent to Cassera, who will test them for their antimalarial effectiveness in a variety of cellular and animal models. Max Totrov will bring his expertise in machine-learning to suggest further modifications that would improve in vivo antimalarial efficacy, and reduce mammalian toxicity.
The team is optimistic that their research will yield candidates for advanced pre-clinical evaluation, a process that may require partnership with a global health public-private partnership. The path to drug development can be long, but is often rewarded with fundamental discoveries in disease biology along the way.
Virginia Tech recently filed a patent application for both drug candidates, in addition to awarding the team a proof-of-concept award.
- Written by Kendall Daniels



